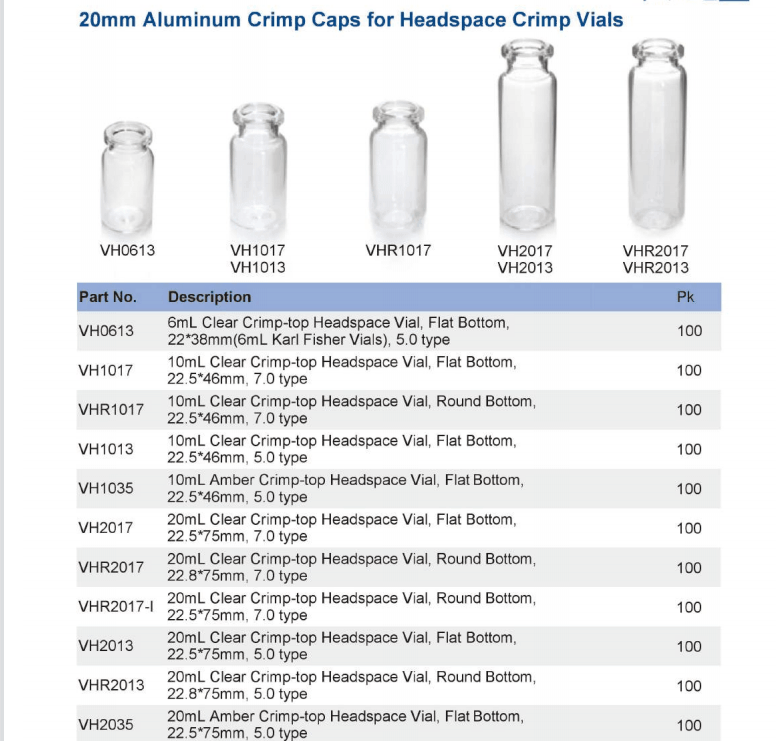
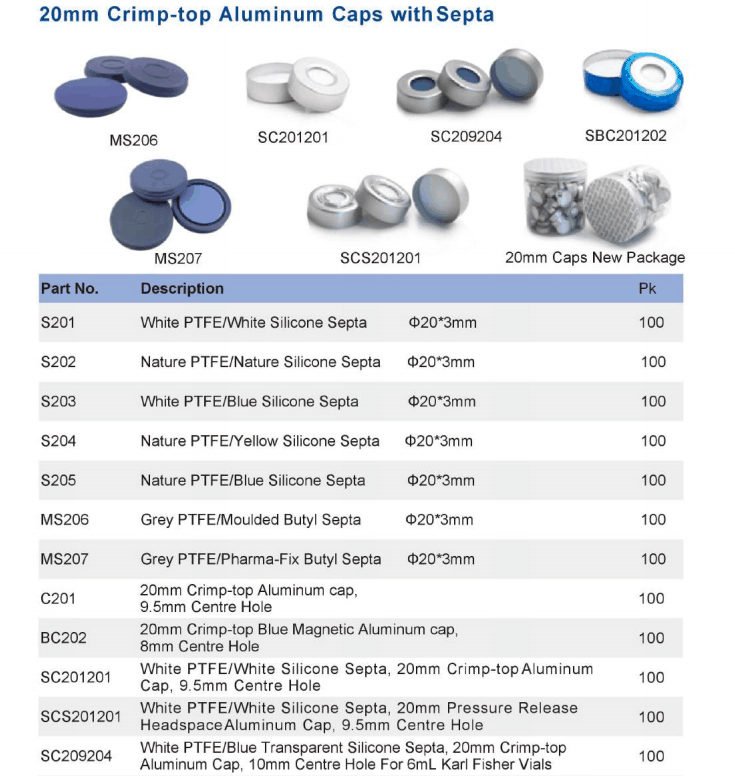
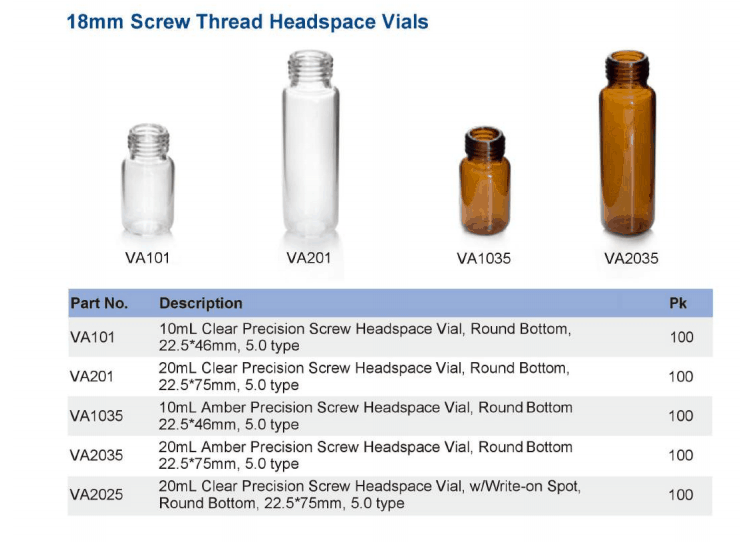
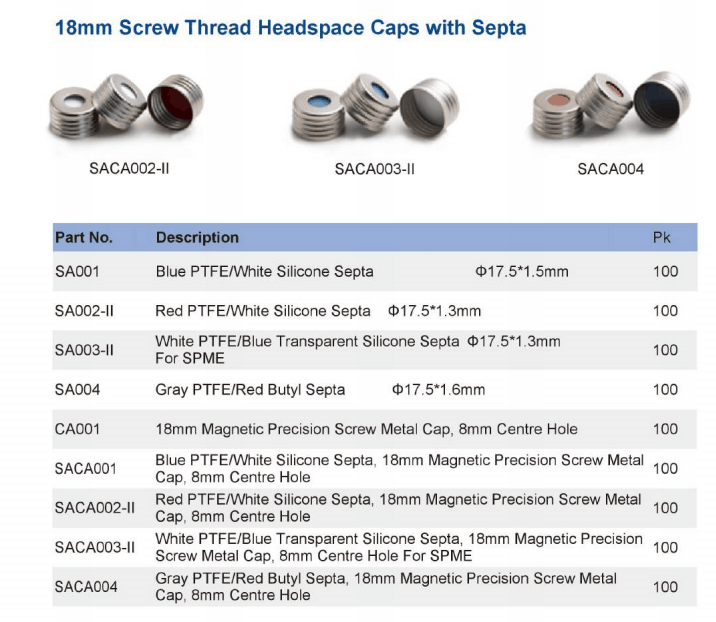
USP Chapter <467>, Residual Solvents, allows pharmaceutical companies the A Teledyne Tekmar Versa Automated Headspace Vial Sampler was connected to a GC ...
Jul 5, 2022 The method is simple and readily adaptable to diversified drug substances ... equilibrated for 10 min at 100° in a 10-mL headspace vial, ...
Oct 18, 2021 Closed system drug-transfer devices (CSTDs) are devices which ... the drug at the same concentration as the headspace inside the vial.
Sep 21, 2020 Nitrosamines Found as Contaminants in Drug Substances and Drug Products ... 1 mL of Standard stock solution to an appropriate headspace vial ...
qualitative analysis using the Polymer Additives Library. 5 μL of an n-alkane solution (1000 μg/mL hexane solvent) was sealed in a headspace vial purged
Results 1 - 30 of 36 SU860029. volume 10 mL, clear glass vial (flat top), flat bottom, O.D. × H × I.D. 22.5 mm × 46 mm × 12.5 mm, long neck. View Pricing ...
Lipid Complex Injection: 10 mg/5 mL (2 mg/mL) in a single-dose vial (3) Anti-drug antibodies to ONPATTRO were evaluated by measuring antibodies specific ...
standard stock solution in an appropriate headspace vial, add exactly 5 mL of test stock solution, and apply the stop- per, cap, and mix.
Test sample: 0.5 g of the drug substances sample was accu- rately weighted into a 10-mL headspace vials, and 2 mL of NMP was added into each vial.
Sep 1, 2020 Traditionally, most drug developers and manufacturers have used 2-mL, 5-mL, and 10-mL clear and amber Type I borosilicate glass for primary ...
ONPATTRO is supplied as a 10 mg/5 mL solution in a single-dose glass vial. quality of the drug. 3. How many vials of ONPATTRO are needed to prepare my ...
3. II. Aspects of drug characterization and impurity profiling . Calibration solutions: Add 5 ml of DISS to four 22-ml headspace vials, then add 50.
Dec 16, 2019 They prove that mechanisms leading to cavitation during drug ... The 5 ml vials were filled with solution volumes of 1, 2, 3 and 4 ml.
in sartan products according to a United States Food and Drug blood pressure.3 ... 20 mL. Vial Shaking. Level 5. Fill Mode. Default. Fill Pressure.
priate selection of the solvent for the synthesis of a drug substance or headspace vial, add 5.0 mL of 1,3-dimethyl-2-imidazolidinone,.

Material: USP Type 1, Class A, 33 Borosilicate Glass
Volume: 2ml (standard volume) 1.5ml(actual volume)
Application: HPLC and GC system
Dimensions: 11.6 x 32mm
Neck Diameter: 8mm
Qty/Pack: 100pcs/pack
Payment: T/T
MOQ: 1pack

Material: USP Type 1, Class A, 33 Borosilicate Glass
Volume: 2ml (standard volume) 1.5ml(actual volume)
Application: HPLC and GC system
Dimensions: 11.6 x 32mm
Neck Diameter: 9mm
Qty/Pack: 100pcs/pack
Payment: T/T
MOQ: 1pack

Material: USP Type 1, Class A, 33 Borosilicate Glass
Volume: 2ml (standard volume) 1.5ml(actual volume)
Application: HPLC and GC system
Dimensions: 11.6 x 32mm
Neck Diameter: 10mm
Qty/Pack: 100pcs/pack
Payment: T/T
MOQ: 1pack